New advance could be the Future of COVID testing. (Source: Nanopore Technologies)
Nanopore Tech’s New Tool to Fight COVID-19 Could Be Game Changer
Oxford-based Nanopore Technologies reports it is close to developing a new generation of the end-to-end test for the detection of SARS-CoV-2, the virus that causes COVID-19, using a desktop sequencer, a palm-sized sequencer and cloud technology. According to a story on news.sky.com, the technology could be ready soon.
The test could make it possible to get results in as little as one hour.
The UK-based company developed the test, called LamPORE, as a rapid, low cost and scalable means of providing on-demand analysis of smaller sample numbers, to very large numbers of samples. Hardware and reagents are outside of the usual supply chains for other molecular tests, which could make it easier to distribute.

The number of samples that can be sequenced in a mobile setting could have a huge impact on the testing or lack of it in the U.S., according to a CNN.com report.
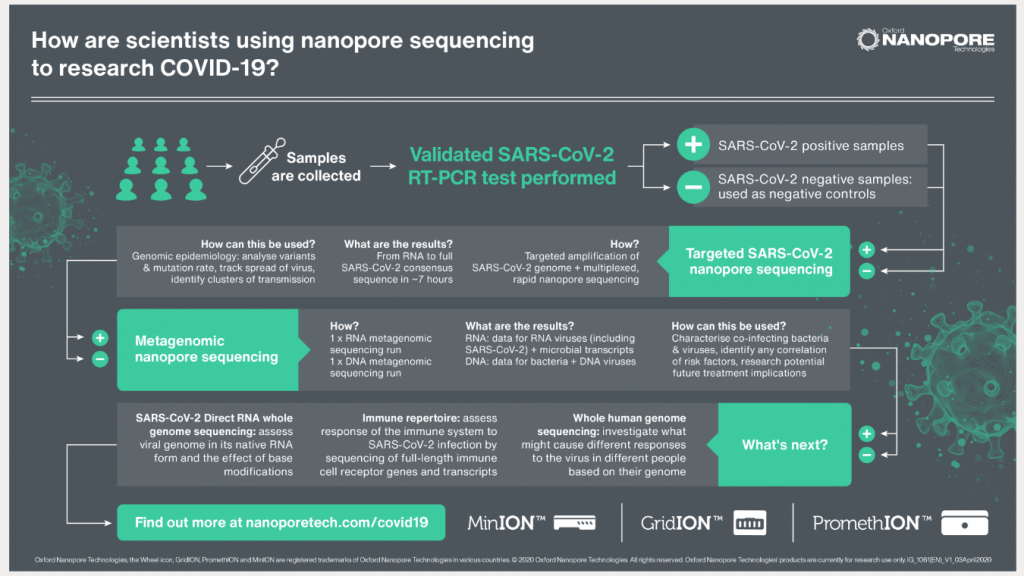
Since the first emergence of the virus, Oxford Nanopore’s rapid, portable sequencing technology has been used extensively for COVID-19 epidemiology and scientific research.
“This year we have gained extensive experience supporting customers using our sequencing technology for COVID-19 epidemiology, and we have also been directing our own powerful innovation and development engine at COVID-19,” said Dr. Gordon Sanghera, CEO of Oxford Nanopore. “We designed our first nanopore sequencing device to be used by any scientist, anywhere. Now, with LamPORE, we want to bring the accessibility and scalability of nanopore sequencing into the area of rapid testing,”
LamPORE can be scaled to process very large sample numbers—a single desktop GridION device can process up to 20,000 samples a day. Additional ultra-high-throughput capacity to existing testing programs has the potential to expand testing from key workers or those with symptoms, to enable broader, regular screening of communities and support an easing of COVID restrictions for the benefit of broad populations.
If you are in the scientific or medical fields, the ACS NANO 2020 review below is filled with testing results and other information.
read more at nanoporetech.com
medical review of COVID pubs.acs.org/doi/pdf/10.1021/acsnano.0c02624






Leave A Comment